Poison Control: Death by Procainamide: Medication Errors and Toxicity
Procainamide is a class 1A antiarrhythmic used for ventricular arrhythmias. A case report by Croskerry and colleagues described an error of communication in transcription for a patient in the emergency department (ED) receiving procainamide for ventricular tachycardia. In this patient case, the intended dose was intravenous (IV) procainamide 100 mg in 10 mL dextrose 5% (D5W) or normal saline (NS). The procainamide formulation supplied in the ED was a vial containing 1000 mg in 10 mL. The nurse withdrew 10 mL of this formulation, for a total of 1000 mg. Prior to administration, the nurse confirmed with the physician that the “whole thing” was to be provided. The physician assumed the prescribed dose of 100 mg in 10 mL was being given, and agreed “yes.” Ultimately, this error led to administration of IV procainamide 1000 mg over three separate instances during the acute resuscitation. The patient subsequently became hemodynamically unstable, was intubated, and died several days later.
This is an example of a miscommunication transmitted verbally to expedite medication administration in the high-stress ED environment. Contributing factors noted were unfamiliarity with the drug, time pressures, and low quality transfer of information. [1] This product was meant to undergo dilution prior to administration, and the patient ultimately received 10 times the intended procainamide dose.
The procainamide package insert recommends procainamide as a direct IV injection or IV infusion for life-threatening ventricular arrhythmias. Direct IV injections of procainamide should be administered at a rate of 100 mg every 5 minutes, up to maximum rate of 50 mg per minute, until the arrhythmia is suppressed or 500 mg has been provided, at which point further doses are to be held for 10 minutes to allow redistribution. IV infusions of procainamide should be administered at 20 mg per minute, for up to 25-30 minutes, to deliver a total of 500-600 mg. [2-3] The maximum dose of procainamide for each of these loading dose techniques is 1000 mg. Additionally, the 2020 American Heart Association (AHA) algorithm for Adult Tachycardia with a Pulse recommends IV procainamide for stable wide-QRS complex tachycardia. The AHA recommends procainamide at a rate of 20 to 50 mg per minute until arrhythmia is suppressed, hypotension ensues, QRS duration increases greater than 50%, or a maximum dose of 17 mg per kg is given. [4] Furthermore, in the PROCAMIO trial, procainamide was dosed as 10 mg per kg, infused over 20 minutes, for wide-complex tachycardia. [5]

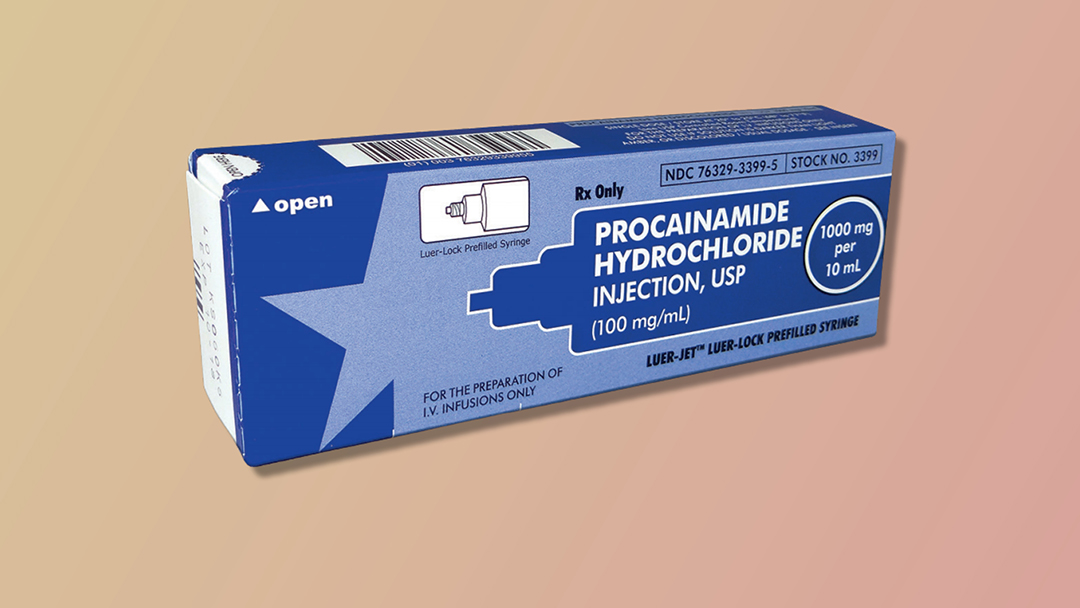
Figure 1. Procainamide Vial (1000 mg per 10 mL)
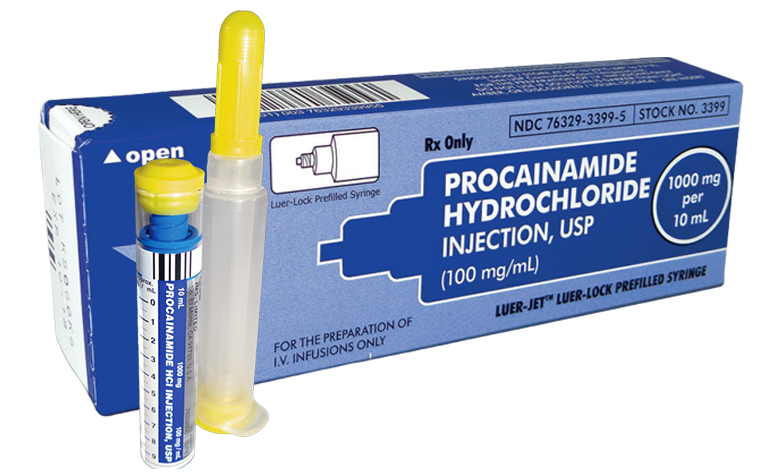 Figure 2. Procainamide Prefilled Syringe (1000 mg per 10 mL)
Figure 2. Procainamide Prefilled Syringe (1000 mg per 10 mL)
Although the package insert, AHA guidelines, and the PROCAMIO trial provide guidance for procainamide dosing, these sources do not address the preparation for administration. Procainamide is available as 1000 mg per 10 mL vials (Fig. 1) and 1000 mg per 10 mL prefilled syringes (Fig. 2). [2-3] Both formulations are to be diluted prior to being given in order to facilitate control of the dosage rate, which is 20-50 mg per minute. Dilution may be achieved by placing the 1000 mg per 10 mL concentration in 50 mL to 250 mL of D5W. Procainamide prefilled syringes carry specific marking on the package label stating: “FOR THE PREPARATION OF IV INFUSIONS ONLY.” [3] These prefilled syringes are manufactured with a luer lock connector typically associated with direct IV administration. Another medication packaged similarly is epinephrine 1 mg in 10 mL (Fig. 3). However, this formulation of epinephrine is intended for direct IV administration and the prefilled syringe of procainamide is not. This procainamide formulation could result in unintentional administration of an undiluted procainamide 1000 mg bolus, despite package labeling stating otherwise. The risk of this error has resulted in specific safety recommendations from the Institute for Safe Medication Practices (ISMP) regarding procainamide prefilled syringes. ISMP recommends stocking procainamide prefilled syringes only in the pharmacy for use to prepare infusions, and stocking procainamide vials in crash carts and emergency supply areas. [6]
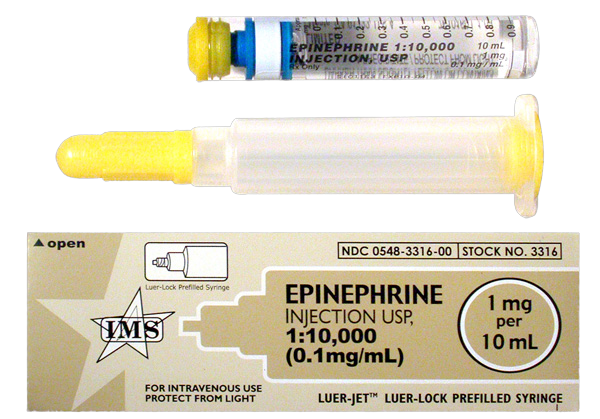
Figure 3. Epinephrine Prefilled Syringe (1 mg per 10 mL)
Dosing errors may result in toxicity due to prolonged AV conduction or AV block. Symptoms of toxicity may occur following a single dose of 2000 mg. Other effects that may occur with severe toxicity are seizures, hypotension, QRS and/or QTc prolongation, ventricular dysrhythmias and respiratory depression. [7] Monitoring parameters should include vital signs, mental status, serial ECGs, serum potassium and magnesium, and renal function. All patients should be placed on continuous cardiac monitoring and managed according to symptoms. This includes treatment of unstable tachyarrhythmias with cardioversion, hypotension with fluid resuscitation and/or inotropic medications, and torsade de pointes with magnesium. Additionally, sodium bicarbonate 1 to 2 mEq/kg should be provided intravenously for wide-QRS complex tachycardia as it may narrow the QRS complex by overcoming the procainamide-induced fast sodium channel blockade. Other treatments include benzodiazepines for seizures and pacemaker placement for increasing AV block. [7]
In order to avoid procainamide-related medication errors and subsequent toxicity, procainamide should be stored in accordance with ISMP recommendations. Procainamide vials should be stored in patient care areas, while procainamide prefilled syringes should be stored in the pharmacy for the preparation of IV infusions. Medical personnel should remember that procainamide requires dilution and the initial loading dose should not exceed the package size. Patients with potential toxicity should be carefully monitored for cardiac adverse events and management will involve symptomatic and supportive care.
Your local poison center is available at 800-222-1222 for any questions regarding procainamide toxicity or to provide assistance with any toxic exposure.
References
- Croskerry P, Shapiro M, Campbell S, LeBlanc C, Sinclair D, Wren P, Marcoux M. Profiles in patient safety: medication errors in the emergency department. Acad Emerg Med. 2004;11(3):289-99.
- Procainamide Hydrochloride Injection, USP [package insert]. Lake Forest, IL: Hospira Inc.; 2021.
- Procainamide Hydrochloride Injection, USP [package insert]. South El Monte, CA: International Medication Systems, LTD.; 2016.
- Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, Kudenchuk PJ, Kurz MC, Lavonas EJ, Morley PT, O’Neil BJ, Peberdy MA, Rittenberger JC, Rodriguez AJ, Sawyer KN, Berg KM; Adult Basic and Advanced Life Support Writing Group. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S366-S468.
- Ortiz M, Martín A, Arribas F, Coll-Vinent B, Del Arco C, Peinado R, Almendral J; PROCAMIO Study Investigators. Randomized comparison of intravenous procainamide vs. intravenous amiodarone for the acute treatment of tolerated wide QRS tachycardia: the PROCAMIO study. Eur Heart J. 2017 May 1;38(17):1329-1335.
- Institute for Safe Medication Practices (ISMP) Action Agenda. (July-September 2017). ISMP Quarterly Action Agenda. Retrieved from https://www.ismp.org/acute-care/july-september-2017.
- Procainamide. In: POISINDEX Managements [database on the Internet]. Greenwood Village (CO): IBM Corporation; 2021 [updated 2020 Sept 3, cited 2021 Mar 7].
This article is part of the following sections: