Bilateral Pneumothoraces After Trigger Point Injection Therapy: A Case Report
ABSTRACT
Introduction Trigger point injections (TPIs) are a common, seemingly benign treatment for myofascial pain. One possible, but rare, adverse event associated with TPI is pneumothorax. To our knowledge, this is the first reported case of TPI-associated bilateral pneumothoraces. Case Report A 30-year-old male with a history of chronic upper back pain received bilateral trapezius TPIs at an interventional pain clinic. After each injection, he developed ipsilateral pleuritic chest pain and shortness of breath. Chest radiographs obtained in the emergency department confirmed the presence of bilateral pneumothoraces requiring urgent bilateral thoracostomies. After five days of hospitalization, the patient was discharged in stable condition. Identified risk factors include thin body habitus and high-risk injection site. Conclusion Although there have been 4 reports of bilateral pneumothoraces after acupuncture, there have been no reports of bilateral pneumothoraces after TPI. As TPIs become more common, clinicians must consider high-risk, anatomic regions and patient-specific risk factors before proceeding with this invasive procedure. Moreover, patients and clinicians must recognize the signs and symptoms of pneumothorax to expedite diagnosis and treatment of this potentially life-threatening condition. Keywords: Trigger point injection, Procedural complications, Iatrogenic pneumothorax, Case reportINTRODUCTION
Trigger points are tight bands of skeletal muscle that can irritate nearby nerves, causing myofascial pain and decreased mobility.[1] While treatment generally consists of conservative, non-invasive modalities, trigger point injection (TPI) is commonly considered for the management of myofascial pain.[1] TPI involves either palpation or ultrasound localization of a trigger point, followed by the injection of an anesthetic, steroid, combination, or neither.1 Pneumothorax is a serious medical condition that occurs when air collects within the thoracic cavity. Left untreated, air may continue to accumulate in the pleural space, resulting in increased intrathoracic pressure, decreased venous return, and ultimately, cardiac arrest. Pneumothorax is an uncommon complication from TPI. Of 5,475 anesthesia malpractice claims included in the American Society of Anesthesiologists Closed Claims Project, 15 involved pneumothoraces secondary to TPI. Most pneumothoraces were diagnosed after a latent period, suggesting this condition to be an underreported event.[2] We present a case of bilateral pneumothoraces after TPI. To our best knowledge, a similar case has not been reported in the literature.CASE REPORT
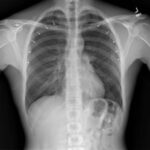
A 30-year-old male presented to our emergency department (ED) with complaints of pleuritic chest pain and dyspnea. His medical history consisted of upper back pain from a previous car accident. He was recently referred to a pain specialist by his chiropractor to explore additional interventions for pain management . On the day of his presentation, he visited an outpatient interventional pain specialist who administered bilateral trapezius TPIs with lidocaine for his upper back pain. The patient was given the impression that TPI had negligible risk, “similar to a standard vaccine injection.” The patient was unaware of the risk of pneumothorax. The trigger points were identified by palpation. Immediately after the first TPI, the patient experienced oropharyngeal paresthesia, dysgeusia, and anterior pleuritic chest pain. The clinician proceeded with the contralateral TPI after conferring with the patient. He experienced similar symptoms immediately after the contralateral TPI, and his symptoms worsened with time. He immediately presented from the clinic to the ED for these symptoms by private vehicle. While in the ED waiting room, the patient received standing triage orders, implemented when our ED is at capacity. These triage orders included serum chemistries, serum blood counts, cardiac enzymes, electrocardiogram (ECG), and plain film radiographs of the chest. While waiting for a room, the radiologist called to report that the patient had bilateral pneumothoraces (Image 1). The patient was placed in a room immediately once the pneumothoraces were identified.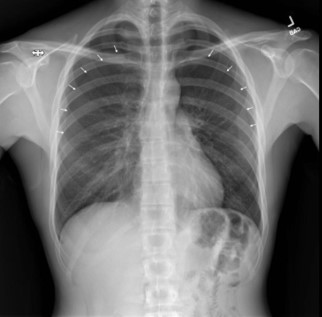
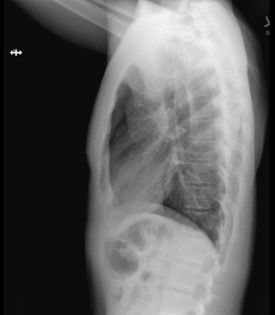
Image 1 – Plain film radiographs of the chest on presentation. Left: Anteroposterior chest view showing bilateral, moderate-size pneumothoraces (arrows) without tension component. Right: lateral view.
Upon initial evaluation, the patient was a young, thin, anxious-appearing male. He reported nausea, back pain, non-productive cough, pleuritic chest pain, and dyspnea. He was noted to be hemodynamically stable with a normal heart rate (74 beats per minute [bpm]) and blood pressure (102/58 mmHg), along with a normal respiratory rate (18 breaths per minute) and oxygen saturation (100% on room air). He was 1.67 m tall and weighed 56 kg. His trachea was midline with no evidence of jugular venous distention. He was speaking in complete sentences. He was noted to have decreased bilateral breath sounds. ECG was normal with a normal rate and sinus rhythm. He was immediately placed on 100% oxygen via non-rebreather mask and informed consent was obtained for bilateral tube thoracostomy.
Systemic analgesia and anxiolysis, along with local anesthesia, was administered prior to tube thoracostomy. Bilateral 16 Fr Thal-Quick® chest tubes were placed without any complications. Post-procedure vitals remained stable (heart rate 51 bpm, respiratory rate 14 breaths per minute, 144/78 mmHg, 100% SpO2). Post-procedural chest plain films showed re-expansion of both lungs (Image 2). The patient also reported clinical improvement of his symptoms. The labs that were sent from the waiting room were within normal limits.
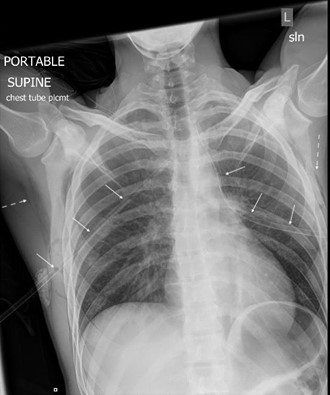
Image 2 – Portable chest radiograph status post bilateral chest tube thoracostomies confirms appropriate placement of bilateral thoracostomy tubes (solid arrows) and re-expansion of both lungs with residual apical pneumothoraces. New bilateral subcutaneous emphysema (dashed arrows) is also evident.
The patient was admitted to the hospital, and pulmonology was consulted. The left-sided tube thoracostomy was placed on a water seal on day 2, and clamped and removed on day 3. The right-sided pneumothorax was larger; therefore, pulmonology recommended water seal on day 4, and the tube thoracostomy was clamped and removed on day 5. There were no observed air leaks during the patient’s hospitalization. The patient was discharged on day 5 with no untoward events.
DISCUSSION
While it is unclear how common TPI is performed in the United States, it is estimated that 30-85% of patients presenting to pain clinics have myofascial pain, for which TPI is a common therapy.[3] Moreover, the emergency medicine literature has recently seen an increase in the amount of published literature concerning TPI, and TPI will likely become increasingly common in the ED.[4,5] Although TPI is generally a safe procedure, it is invasive and has the potential for adverse events. Adverse effects of TPI include nerve or muscle injury, syncope, excessive bleeding, and infection.[6] Rare but severe adverse effects include pneumocephalus, hypokalemic paralysis, and pneumothorax.[7,8,9]
Pneumothorax is defined as the collection of gas in the pleural space and is a medical emergency that frequently requires immediate intervention. Iatrogenic pneumothorax occurs as a result of a diagnostic or therapeutic medical intervention and has an incidence of 5 per 10,000 hospital admissions.[10] Bilateral pneumothorax is rare, accounting for 1% of all pneumothorax cases in the literature.[11]
Pneumothoraces secondary to invasive, iatrogenic procedures are often associated with thoracentesis and central venous catheterization. One study found that in patients undergoing thoracentesis, being underweight (BMI [body mass index] < 18.5) significantly increased the risk of iatrogenic pneumothorax. While it is unclear whether this association translates to TPI, it seems intuitive that the risk of iatrogenic pneumothorax is higher in patients with thinner body habitus.[12] Our patient approached the underweight threshold with a BMI of 20.1.
While previous case reports have reported on TPI-associated iatrogenic pneumothorax,[7] our case is unique, and several key points are worth further discussion. First, TPI-associated iatrogenic pneumothorax is a relatively uncommon complication. According to the American Society of Anesthesiologists Closed Claim Project, out of 5,475 anesthesia malpractice claims, only 15 involved pneumothoraces secondary to TPI.[2] Similarly, there is a paucity of literature concerning this complication, likely due to its infrequent occurrence. Although pneumothorax occurs infrequently after TPI, it is a potentially life-threatening condition, and clinicians must be aware of and recognize this potential complication.
Second, to the authors’ best knowledge, no reports of bilateral pneumothoraces secondary to TPI were found in the literature. Although it is naturally expected that bilateral needling can result in bilateral pneumothoraces, the clinician did not recognize the signs and symptoms of potential iatrogenic pneumothorax before proceeding with the contralateral TPI. Consequently, other potential reasons for its low reported frequency in the literature include lack of recognition, difficulty in data collection, delays in diagnosis, or spontaneous resolution of small pneumothoraces, several of which are evidenced in our presented case.[5] Of interest to our case is the practice of acupuncture, which involves the insertion of needles into specific parts of the body for therapeutic purposes, and, similar to TPI, is commonly used to manage myofascial pain syndrome.[13] The authors found four cases of bilateral pneumothoraces secondary to acupuncture in the literature, warranting discussion of possible factors associated with adverse events and poor outcomes.[14] Needle thickness and length used during acupuncture and TPI can vary significantly, but those used for TPI are generally similarly sized or larger. While some advocate for imaging guidance, both practices commonly employ needle insertion based on palpation alone. Of the four cases of bilateral pneumothoraces secondary to acupuncture, two cases resulted in patient death. Each patient had varying body habitus, and notably, each case involved injection in the cervicothoracic region of the body.[14]
TPI administered in the cervicothoracic region is common, and there is a higher reported incidence of pneumothorax associated with this injection region; caution should be exercised when performing TPI in this area.[7] One of the limitations of this report is the exact needle length used for our patient’s TPI is unknown; the patient reported the needle was “very long” and approximately the length of his index finger. Given the patient’s thin body habitus and cervicothoracic injection site, the authors believe this patient was at increased risk of iatrogenic pneumothorax. We speculate the needle was inserted too deeply in this patient, resulting in bilateral pneumothoraces. The authors are not critiquing the decision-making process or technical skills of the proceduralist; rather, we present this case to remind clinicians and patients of risk factors associated with TPI.
Third, the patient did not receive adequate informed consent. He was not aware of what the procedure entailed and the potential complications and adverse effects. One could speculate that if he knew the potential complications from TPI, the contralateral pneumothorax may have been averted.
Finally, as TPI becomes more widely available in family medicine practices and in the emergency department, it is incumbent on physicians to be aware of this associated complication and its risk factors to prevent it from occurring. It is also important to recognize its signs and symptoms so that treatment can be delivered expeditiously.
In order to prevent future complications, it is imperative that clinicians carefully consider a patient’s body habitus, unique risk factors, depth of injection, and injection site when performing invasive procedures. Some studies advocate for imaging guidance during procedures in the higher-risk cervicothoracic musculature. Others believe ultrasonography to be the solution for complications related to injection depth, regardless of injection site. While palpation is typically utilized for the initial localization of trigger points, ultrasonography can be used to guide injection location and depth.[10] Clinicians have advocated for the use of ultrasound during TPI with the belief that its safety and efficacy in other interventions may translate to safer injection techniques and a less-risky alternative than “blind injection.” Additionally, a systematic review conducted by Diep et al. found that all included ultrasound-guided procedures resulted in zero or minimal self-limited adverse effects. Further investigation is warranted into the safety and efficacy of ultrasonography during TPI.[15]
Due to the paucity of cases, case reports such as this serve to remind clinicians of risk factors, the invaluable benefit of ultrasound guidance, and the potential complications of TPI.
CONCLUSION
Although TPI is generally a relatively benign procedure, it can cause serious adverse events which have the potential to be life-threatening if not recognized and treated in an expedient manner. To our knowledge, this is the first reported case of bilateral pneumothoraces secondary to TPI. Our patient had two key risk factors for TPI-associated pneumothorax: thin body habitus and injection location. It is, therefore, imperative for clinicians to determine the presence of these risk factors prior to procedural intervention and for clinicians to consider pneumothorax as a possible complication after TPI.
REFERENCES
1. Appasamy M, Lam C, Alm J, et al. Trigger Point Injections. Phys Med Rehabil Clin N Am. 2022;33(2):307-333.
2. Fitzgibbon DR, Posner KL, Domino KB, et al. Chronic pain management: American Society of Anesthesiologists Closed Claims Project. Anesthesiology. 2004;100(1):98-105.
3. Han SC and Harrison P. Myofascial pain syndrome and trigger-point management. Reg Anesth. 1997;22(1):89-101.
4. American College of Emergency Physicians. ACEP // Trigger Point Injection. https://www.acep.org/patient-care/map/map-trigger-point-injection-tool/. Accessed February 18, 2023.
5. LaPietra A. Trigger Point Injection for Musculoskeletal Pain in the ED. 2018; https://www.aliem.com/trigger-point-injection-musculoskeletal-pain/. Accessed February 18, 2023.
6. Cheng J and Abdi S. Complications of joint, tendon, and muscle injections. Tech Reg Anesth Pain Manag. 2007;11(3):141-147.
7. Ahiskalioglu EO, Alici HA, Dostbil A, et al. Pneumothorax after trigger point injection: A case report and review of literature. J Back Musculoskelet Rehabil. 2016;29(4):895-897.
8. Nelson LS and Hoffman RS. Intrathecal injection: unusual complication of trigger-point injection therapy. Ann Emerg Med. 1998;32(4):506-508.
9. Soriano PK, Bhattarai M, Vogler CN, et al. A Case of Trigger-Point Injection-Induced Hypokalemic Paralysis. Am J Case Rep. 2017;18:454-457.
10. McKnight CL and Burns B. Pneumothorax. StatPearls. Treasure Island: StatPearls Publishing; 2022.
11. Sayar A, Turna A, Metin M, et al. Simultaneous bilateral spontaneous pneumothorax report of 12 cases and review of the literature. Acta Chir Belg. 2004;104(5):572-576.
12. Cho HY, Ko BS, Choi HJ, et al. Incidence and risk factors of iatrogenic pneumothorax after thoracentesis in emergency department settings. J Thorac Dis. 2017;9(10):3728-3734.
13. Gazi MC, Issy AM, Avila IP, et al. Comparison of acupuncture to injection for myofascial trigger point pain. Pain Pract. 2011 2011;11(2):132-138.
14. Mohammad N. Bilateral tension pneumothorax after acupuncture. BMJ Case Rep. 2018;2018.
15. Diep D, Chen KJQ, Kumbhare D. Ultrasound-guided interventional procedures for myofascial trigger points: a systematic review. Reg Anesth Pain Med. 2021;46(1):73-80.
This article is part of the following sections: