Ultrasound Guided Vascular Access Workshop: A DIY Guide for Homemade Phantoms
Background
Vascular cannulation is a fundamental clinical skill that is applied across diverse medical specialties. Insertion of central venous catheters (CVC) is a common procedure to establish definitive venous access for infusion of sclerosing or vasoactive medications. While these procedures are relatively straightforward, they do carry major risks particularly in patients with anatomically challenging or abnormal vascular anatomy. [1] To help address these challenges, ultrasound guidance has become the standard of care for establishment of central venous access in the emergency department.
Ultrasound (US) guidance for CVC placement has shown impressive benefits in the reduction of total complication rate, and number of attempts and improved overall success rate. [2] Multiple observational and randomized controlled studies demonstrate improved procedural success rates with the use of ultrasound guidance for the insertion of all manner of vascular access devices. [3,4,5,6]
Current Practice
To keep pace with the expansive use of US-guided procedures, medical education programs must evolve and create new opportunities for simulation-based, inexpensive, no-risk learning programs using low-fidelity models. [7,8] Handmade and/or commercial “phantoms,” tools designed to mimic the sonographic and physical properties of human tissue under ultrasound, have become popular training tools to teach vascular access techniques. [9, 10] After receiving phantom training, operators reported significantly higher comfort using ultrasound compared to those who did not. [6, 7]
Low-cost handmade phantoms using chicken breast, tofu, and gelatin mixtures are utilized in settings where commercial models are not readily available or affordable [11,12] and have been shown to be comparable to commercial phantoms. [13] A recent systematic review of undergraduate medical education revealed that 21 out of 95 formal ultrasound curricula utilized phantoms, eight of which were handmade. [14]
This article details a workshop designed by the University of Miami’s Ultrasound and Anesthesia Interest Groups, facilitated by emergency medicine & anesthesia physician educators. The workshop utilized the advantages of affordable homemade gel phantoms to simulate US-guided procedures for undergraduate medical students in a safe, cost-effective manner. This article will provide the methods, resources, and workshop structure for other student interest groups to reference as a guide to host similar sessions.
Objectives
The main objectives of this two-hour workshop were for students to be able to obtain appropriate views of access sites on human models, gain familiarity with central vessel catheterization kits, and successfully cannulate phantom models under US guidance. Students practiced using the Sonosite SII, Butterfly iQ+, and Philips Lumify ultrasound machines. Each station was led by 1-2 emergency medicine or anesthesia residents. The student-to-machine ratio was ~6:1, with “time-on-probe” estimated at roughly 15 minutes per student per station.
Materials & Methods
Two different central access phantoms were made: one to simulate femoral central line placement and another for subclavian central venous line placement. Materials required to create the gel phantom model are listed in Table 1.
Table 1. Materials for the phantom model construction and the procedure.
| Materials | Tools | |
| Model construction | Knox Gelatin (120 g/L) Psyllium Husk Powder (110g/L) Citric Acid (10g/L) 0.25” ID Latex Tubing 0.33” Diameter Nylon Rope |
18oz Disposable Foam Cups |
| Procedure | Ultrasound Gel Absorbent chucks Central Venous Access Kit Sharps Disposal Bin |
10cc Syringe (empty) Water (colored if desired) Ultrasound Machine Linear Transducer |
To create the femoral access phantom, we suspended two 6-inch lengths of 0.25-inch internal diameter latex tubing within an 18 oz. disposable foam cup using drinking straws. A commercially available 0.33-inch diameter nylon rope was also suspended lateral to the vessels to constitute a simulated femoral nerve (Fig. 1). To create the subclavian venous model, a 3D-printed PLA plastic clavicle was placed above tubing representing the subclavian vein. The tubing was then embedded into a gelatin mixture to construct the simulated central access site.
To create the gelatin mixture, we slowly mixed 120 g of Knox Gelatin (Treehouse Foods Inc, Oak Brook, Illinois, USA) powder into 500 mL of cool water using a power mixer or hand whisk (Fig. 2). Once fully dissolved, we added 500 mL of boiling water to the gelatin mixture while mixing continuously. Then, 110 g of commercially available psyllium husk powder and 10 g of citric acid powder was slowly added to the mixture, and we continued to mix until well combined. We suspended the structures centrally inside a container to allow for a 1-inch-thick gel layer around all sides of the vessel and coated the container with commercially available non-stick cooking spray to aid in removal. The mixture was set overnight in a refrigerator (1.7-3.3oC) for approximately 12 hours. Once the gel had set, we removed and discarded the molds. For the femoral access model, arterial blood flow and pulsatility were simulated by connecting a syringe partially filled with colored water to one end of the latex tubing. The syringe was periodically pushed to create pulsatile flow on color doppler. Students used central line kits to cannulate vessels. The total cost to produce one phantom is approximately $3.88, not including the vascular access kit and ultrasound machine.
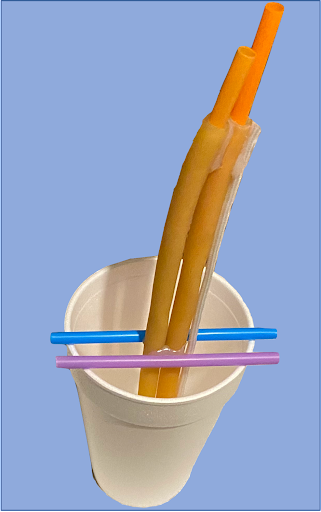

(Left) Fig. 1: The Femoral Access Model mold. Straws were removed once gel had solidified.
(Above) Fig. 2: Gelatin phantom ingredients.
Results
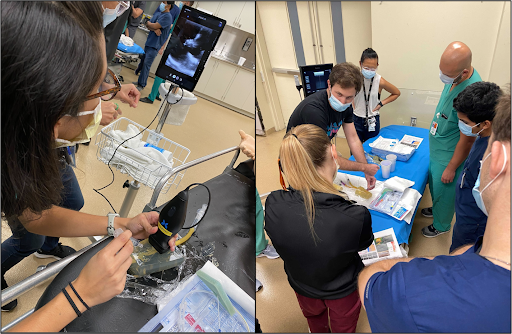
Pre- and post-survey questionnaires were used to assess students’ experience with the workshop. Operators assessed improvement in student comfort with the following tasks on a 1-5 Likert scale: “Setting up the US machine;” “Identifying a Vein and Artery;” and “Using US to insert a Central Line.” All tasks demonstrated statistically significant positive results (p < 0.05), indicating the students felt more comfort with the described tasks after the workshop. Fig. 3 demonstrates real time images from the workshop.
Fig. 3: Model stations and live hands-on instruction.
Discussion
The adoption of simulation models into modern undergraduate medical education has been limited, and lack of financial support may be one of the prevailing barriers in doing so. [14, 15]
The phantoms used in this workshop demonstrate how low-cost models can be effectively utilized for undergraduate ultrasound medical education. While there is no consensus on criteria to assess ultrasound competency, [15] early exposure to develop comfort with materials and technical skills may be beneficial. Medical students participating in this workshop reported a significant improvement in handling an ultrasound probe, satisfaction with understanding the benefit of bedside use of ultrasound, and readiness to use ultrasound in a clinical setting. Our workshop provides a foundation upon which medical students can build their clinical knowledge. ■
References
- Silverman MA. Immune 1) Rippey JC, Blanco P, Carr PJ. An affordable and easily constructed model for training in ultrasound-guided vascular access. J Vasc Access. 2015 Sep-Oct;16(5):422-7. doi: 10.5301/jva.5000384. Epub 2015 Sep 1. PMID: 26349885.
- Saugel, B., Scheeren, T.W.L. & Teboul, JL. Ultrasound-guided central venous catheter placement: a structured review and recommendations for clinical practice. Crit Care 21, 225 (2017). doi.org/10.1186/s13054-017-1814-y
- Egan G, Healy D, O’Neill H, et al. Ultrasound guidance for difficult peripheral venous access: systematic review and meta-analysis. Emerg Med J. 2013;30(7):521-526. doi.org/10.1136/emermed-2012-201652.
- Smith CC, Huang GC, Newman LR, et al. Simulation training and its effect on long-term resident performance in central venous catheterization. Simul Healthc J Soc Simul Healthc. 2010;5(3):146-151. doi.org/10.1097/ SIH.0b013e3181dd9672.
- Dodge KL, Lynch CA, Moore CL, Biroscak BJ, Evans LV. Use of ultrasound guidance improves central venous catheter insertion success rates among junior residents. J Ultrasound Med. 2012;31(10):1519-1526. doi. org/10.7863/jum.2012.31.10.1519.
- Andreatta P, Chen Y, Marsh M, Cho K. Simulation-based training improves applied clinical placement of ultrasound-guided PICCs. Support Care Cancer Off J Multinat Assoc Support Care Cancer. 2011;19(4):539-543. doi.org/10.1007/s00520-010-0849-2
- Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260-273. doi.org/10.7326/0003-4819-142-4-200502150- 00008
- O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Raad II, Randolph A, Weinstein RA. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2002 Aug 9;51(RR-10):1-29. PMID: 12233868.
- Di Domenico S, Santori G, Porcile E, Licausi M, Centanaro M, Valente U. Inexpensive homemade models for ultrasound-guided vein cannulation training. J Clin Anesth. 2007 Nov;19(7):491-6. doi: 10.1016/j.jclinane.2007.05.002. PMID: 18063202.
- Selame LA, Risler Z, Zakaria SJ, Hughes LP, Lewiss RE, Kehm K, Goodsell K, Kalwani R, Mirsch D, Kluger SB, Au A. A comparison of homemade vascular access ultrasound phantom models for peripheral intravenous catheter insertion. J Vasc Access. 2021 Nov;22(6):891-897. doi: 10.1177/1129729820961941. Epub 2020 Oct 6. PMID: 33023394.
- Cheruparambath V, Sampath S, Deshikar LN, Ismail HM, Bhuvana K. A low-cost reusable phantom for ultrasound-guided subclavian vein cannulation. Indian J Crit Care Med. 2012 Jul;16(3):163-5. doi: 10.4103/0972-5229.102097. PMID: 23188960; PMCID: PMC3506077.
- Chao SL, Chen KC, Lin LW, Wang TL, Chong CF. Ultrasound phantoms made of gelatin covered with hydrocolloid skin dressing. J Emerg Med. 2013 Aug;45(2):240-3. doi: 10.1016/j.jemermed.2012.11.022. Epub 2013 Feb 8. PMID: 23399392.
- Lahham S, Smith T, Baker J, Purdy A, Frumin E, Winners B, Wilson SP, Gari A, Fox JC. Procedural simulation: medical student preference and value of three task trainers for ultrasound guided regional anesthesia. World J Emerg Med. 2017;8(4):287-291. doi: 10.5847/wjem.j.1920-8642.2017.04.007. PMID: 29123607; PMCID: PMC5675970.
- Davis, J.J., Wessner, C.E., Potts, J., Au, A.K., Pohl, C.A. and Fields, J.M. (2018), Ultrasonography in Undergraduate Medical Education: A Systematic Review. J Ultrasound Med, 37: 2667-2679. doi.org/10.1002/jum.14628
- Díaz-Gómez, J. L., Mayo, P. H., & Koenig, S. J. (2021). Point-of-Care Ultrasonography. New England Journal of Medicine, 385(17), 1593-1602.
This article is part of the following sections:




