Disruptive Innovation in Emergency Medicine 2.0
Every year at ACEP, the exhibit hall features IncubatED: a medical device innovation challenge. This is an interactive, educational space that provides a sneak-peek of new innovations in emergency medicine. This last year, four medical device start-up companies participated virtually in a “Shark Tank” style pitch competition. This article highlights their disruptive technologies.

Hyperfine Research – Portable MR Imaging Technology
It is not uncommon for patients to present to the emergency department (ED) with complaints of headache, dizziness, numbness, weakness or vertigo. However, despite 40+ years since its inception, there are still a number of barriers to using magnetic resonance imaging in the ED. Many departments lack an in-house MRI scanner secondary to cost and space requirements. For institutions that can afford one, it is often located far from ED patient care areas and is backlogged for hours on waiting scans. This results in long holding times, significant throughput issues, and decreased utilization. Clinicians are often forced to rely on lower accuracy, radiation-producing CT scanners as an alternative.
Hyperfine Research, Inc. is developing a new, portable, easy-to-use, and affordable MRI scanner that is small enough to be wheeled into individual patient rooms. While the fundamental principles of MRI technology and its components still exist, they have leveraged the million-fold improvement in computing power since the first system was designed almost 40 years ago. This results in the machine generating high-quality images while using only 2-6% of the typical MRI magnetic field. The device is also 10 times lighter and uses 35 times less energy to run. Hyperfine secured initial FDA clearance in 2020, and early this year secured Series D funding to scale up commercial rollout of the device. This new portable, functional MRI machine could be a game changer for improving patient care, throughput, and access to MR technology in the emergency department.
For more information, visit: https://hyperfine.io

Coagulo Medical Technologies – Precision Coagulopathy Testing
Approximately 10 million people in the U.S. are on anticoagulation medications, which are a common cause of drug-related adverse events. Oral anticoagulants are common, and Direct Oral Anticoagulants (DOACs) account for more than 50% of oral anticoagulant prescriptions. Despite widespread use, there is currently no FDA-approved clinical test to assess for levels or efficacy of these drugs. Current coagulation testing like the INR is based on 50-year-old technology that provides only limited general coagulopathy information. This puts patients at unnecessary risk of adverse bleeding events, especially in the emergent and surgical settings.
Coagulo Medical Technologies, an MIT-born startup, has developed the world’s first precision-medicine platform for comprehensive and targeted blood clotting management. Their device is capable of pinpointing abnormalities across the entire clotting cascade and delivering clinically-valuable information that no other technology provides. This is accomplished using an entirely new set of patent-pending coagulation assays, microfluidic technology, and proprietary data analytics. Coagulo’s new point-of-care testing platform is the first of its kind to deliver accessible and personalized diagnosis and management of all coagulation-related diseases. It requires only a few drops of whole blood, delivers results within 10 minutes, is fully automated and portable, and can perform up to 20 tests simultaneously. In a 100-patient blinded clinical study performed at the Massachusetts General Hospital Emergency Department, Coagulo demonstrated 100% specificity and over 95% sensitivity for identifying patients’ DOACs. This could enable clinicians to more rapidly and safely control bleeding. In the ED, where minutes can mean the difference between life and death, a rapid, portable, easy to use coagulation assay for detecting DOACs could be, well, life-changing.
For more information, visit: https://www.coagulomed.com

EM Device Labs – QuickloopTM Abscess Treatment Device
Abscess drainage is necessary in over 1.4 million ED patients in the U.S. every year. Despite its frequency, it is still the second most painful procedure commonly performed in the ED. Abscess evaluation is consistently the seventh most common chief complaint for presenting ED patients every year. Loop placement is quickly becoming the industry standard, with several studies showing lower failure rates and improved patient satisfaction, cosmetic appearance, and overall cost of care. Despite this evidence, there is no dedicated loop device.
EM Device Labs have developed the QuickloopTM Abscess Treatment Device, a patented, single-use loop with introducer and securing mechanisms. This device is composed of a curved needle, cutting bevel, and tubing with irrigation fenestrations. It also has a locking mechanism that eliminates the need for knotting the tubing. The QuickloopTM is designed to simplify the “loop” technique, improve outcomes, reduce provider time, and decrease the cost of caring for patients with abscesses. In addition, it incorporates the ability to irrigate the abscess from the inside out with a standard luerlock syringe. Given the potential to improve patient care and ease of use, as well as reduce discomfort and complications, the EM Device Lab QuickloopTM may be a useful device to incorporate into your abscess drainage care.
For more information, visit: http://www.emdevicelab.com
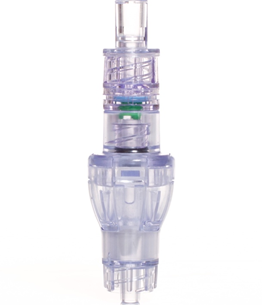
Lineus Medical – Safebreak® Vascular
Approximately 60-90% of patients entering the hospital will receive an intravenous catheter, resulting in 220 million peripheral IVs every year in the U.S. However, up to 46% of these IVs will fail prematurely. Failure can be from any number of reasons, including phlebitis, infiltration, occlusion, dislodgement and infection. On occasion, mechanical stress on the line can cause one of these failures. Staff will typically tape and place an adherent dressing to secure the lines in place.
To decrease mechanical failure rates of peripheral IVs, Lineus Medical has developed their Safebreak® Vascular Dislodgement Prevention Device. This patented, FDA clearance-pending device is composed of an IV connected with a pressure sensitive release mechanism that is designed to release if a certain amount of pressure is applied. Upon release and disconnection, both ends seal to prevent loss of blood or infusion liquid. In theory, the quick release mechanism will prevent mechanical dislodgement and failure. This could result in fewer venipunctures, reduce procedure delays, and increase the time nurses can spend on other patient care activities.
For more information, visit: https://lineusmed.com
This article is part of the following sections: