ITP vs. VITT: Rare but Distinct Conditions Associated with the COVID-19 Vaccines
Currently, there are three COVID-19 vaccinations approved by the Food and Drug Administration (FDA) for use in the United States: Pfizer-BioNtech, Moderna and Johnson & Johnson (J&J), with J&J being the only single-dose formulation. While the J&J vaccine uses a traditional adenovirus vector, the other two vaccines are the first vaccines to use long-studied messenger RNA (mRNA) technology to deliver the immunogenic material to the host. The mRNA is translated to an endogenous version to the coronavirus spike protein, which engenders an immune response and is then quickly degraded, prior to being incorporated into the nucleus. This mRNA technology has actually been in existence for many years, employed in the treatment of certain cancers and genetic diseases.
Although production of endogenous spike protein cannot cause an active infection, there has been conflicting and concerning data regarding the dangers of the spike protein itself. Emerging evidence suggests that the spike protein generated by our intramuscular and endothelial cells following administration of the COVID-19 vaccines may up-regulate a pro-thrombotic pathway via platelet factor 4 (PF4); the same molecule known to cause Heparin Induced Thrombocytopenia (HIT). Additionally, it is theorized that the lab-engineered lipid nanoparticle covering of the mRNA may lead to unpredictable absorption rates in the central nervous system, resulting in various neurological side effects.
Since initiating COVID-19 vaccination of healthcare workers and the general population, rare occurrences of anaphylaxis have occurred. As of January 2021, only 21 cases of anaphylaxis have been recorded in the 1.8 million doses of Pfizer-BioNTech vaccine given so far (11 cases per million). What has not been reported by the CDC and the vaccine manufacturers yet, are the unquantified incidence of other life-threatening reactions. Unfortunately, there does seem to be an emergence of rare side effects from these vaccines, centered on the coagulation cascade and resulting in both thrombotic and bleeding events. Although the pathogenesis of the syndrome VITT is not yet fully defined, certain findings have been consistently seen.
In almost every patient with COVID-19 associated VITT, high levels of antibodies to PF4–polyanion complexes were identified. In patients with HIT, heparin binds to PF-4, forming a complex that binds and activates platelets and monocytes, resulting in profound thrombosis and inflammation. In VITT, it is the spike protein that binds to PF-4, resulting in the same platelet activation, thrombosis and destruction. There is also not yet enough data on COVID-VITT to determine mortality and morbidity, but the similarities to the HIT pathway are extremely concerning when one considers the 20% mortality seen in HIT.
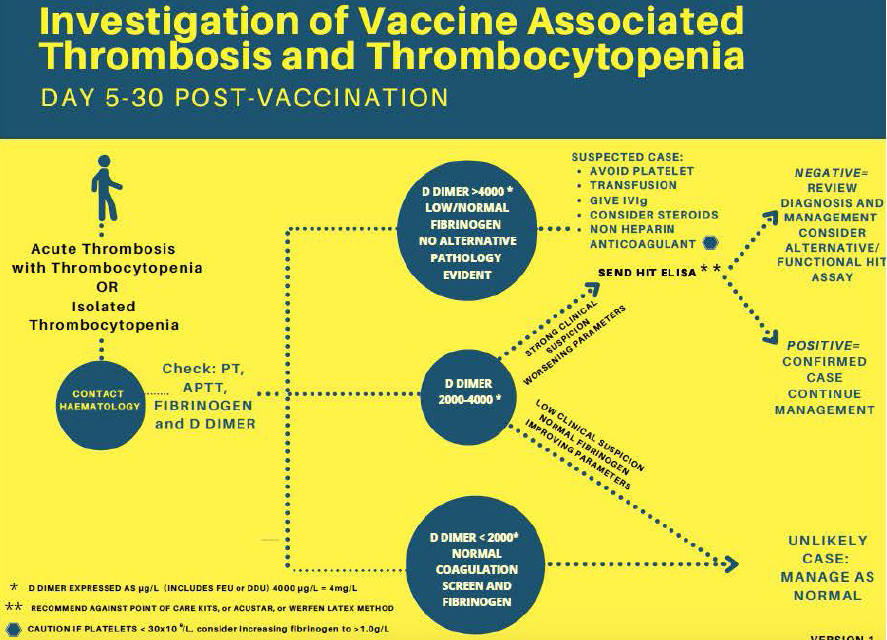
Differentiating between COVID vaccine-induced ITP from VITT can be challenging but the distinction becomes imperative when considering initial management. The key distinguishing feature is the presence or predilection for thrombosis. Clinical signs of thrombosis, however, may be subtle. Fortunately for physicians, there exists an extremely sensitive tool perfectly tasked for this: the D-dimer. In the absence of overt or indirect signs of thrombosis, VITT can be screened by D-dimer levels, which are typically available on a timely basis in any emergency department. D-dimers of >2000 ng/ml with strong clinical suspicion, or dimer of >4000 ng/ml makes a strong case for VITT. Although minor elevations in D-dimer may be seen in ITP, values are typically orders of magnitude lower and thrombosis is rarely seen. If D-dimer is grossly elevated in patients with thrombocytopenia, even those seen up to 42 days post-COVID-19 vaccination, one can reasonably begin treatment for VITT while awaiting more definitive diagnosis. VITT can be subsequently definitively diagnosed with a PF4 ELISA assay, though most emergency clinicians will not have immediate access to this laboratory test. Additionally, one should not rely on the rapid assays that are often used to detect HIT unless they have been specifically validated for VITT, given the potential differences in antigenic target or sensitivity. Fibrinogen levels should also be checked in all patients with suspected VITT. While not recommended for diagnostic criteria, fibrinogen levels may be low in patients with VITT, and therapy should be adjusted accordingly (see Figure 1 and Table 1).
Fig. 1. Diagnosis of Vaccine Induced Thrombotic Thrombocytopenia
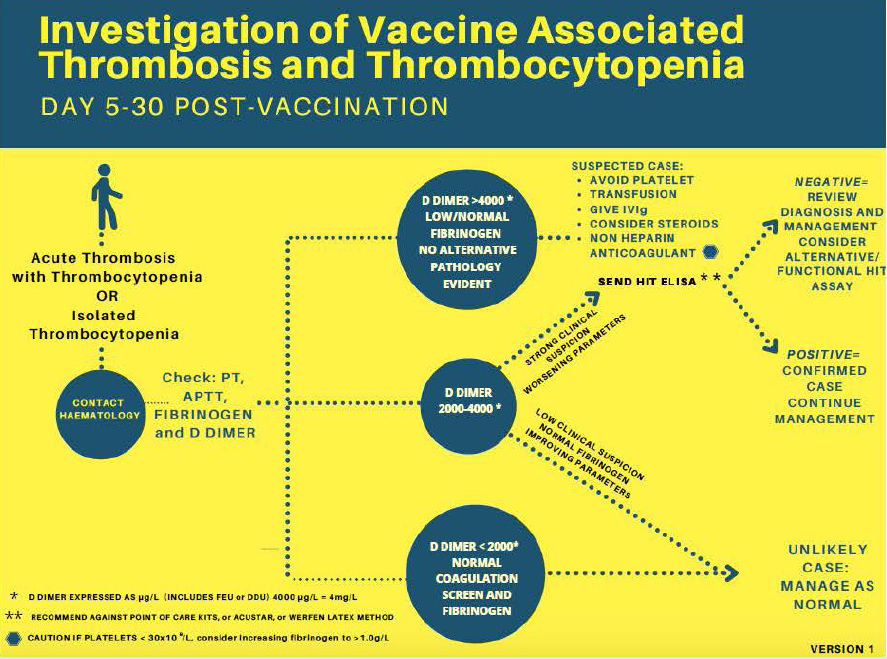
Table 1. Management of COVID-19 Vaccine-Related ITP vs. VITT
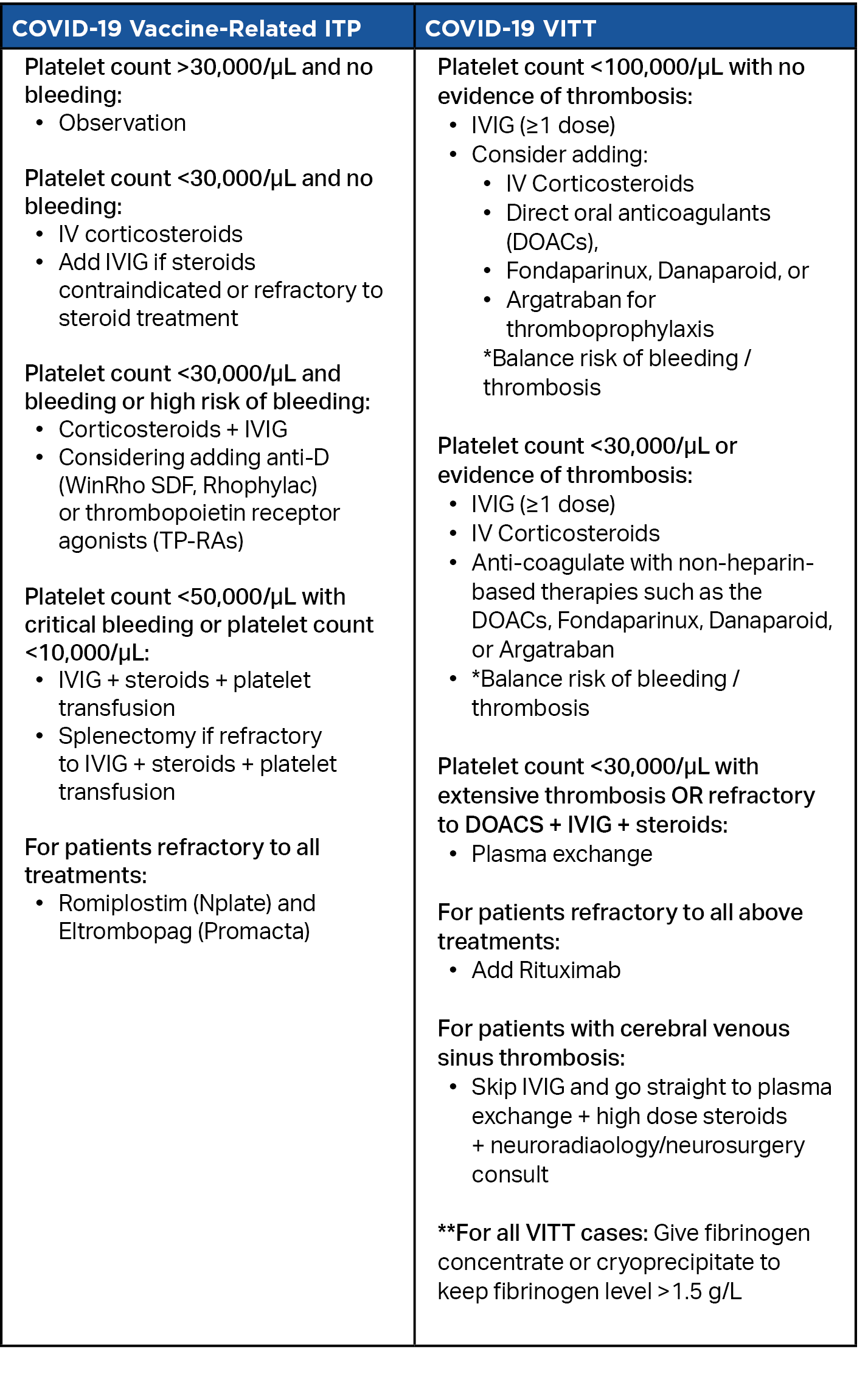
Determining whether a patient has COVID-19 vaccine-induced ITP vs VITT changes the treatment strategy, which in the case of VITT, must target both bleeding and thrombosis, or the risk thereof. As outlined in the table below, treatment algorithms and lab cutoffs for each condition differ in notable ways. There is not yet enough evidence to fully substantiate these treatment options, but the algorithm represents the best current consensus guidelines from experts in hematology, and may provide a good starting point until additional evidence is obtained.
Conclusion
It must be emphasized that despite these rare hematologic complications, the overall safety and benefits of the COVID-19 vaccine are tremendous. The vaccines have already prevented thousands, if not millions of infections, and continue to save many lives. Emerging side effects, while concerning, are extremely rare. Clinicians advising patients on initial vaccination must consider the dangers and susceptibility of their patients to COVID-19 infection weighed against specific risk factors for side effects from the vaccines. Patients with a history of ITP or other hematologic disorders should consult their hematologist. The incidence of symptomatic thrombocytopenia post vaccination is generally well below the risk of death and morbidity from SARS‐CoV‐2 infection.
In the emergency department, most physicians have become all too familiar with the ravages of infection by SarsCoV2. It behooves all emergency clinicians to also learn about the serious (albeit rare) complications of COVID-19 vaccination. As with most studies and findings related to the novel virus, much more research is needed to establish more categorical guidelines and recommendations regarding VITT and other post-vaccination phenomena. There have not been nearly enough cases to definitively substantiate the interventions above, but the algorithms represent the best guidance currently available from experts in hematology. It is critically important that emergency physicians are aware of the distinct entity of VITT to initiate a proper diagnostic and therapeutic strategy. In the absence of much-needed additional data and randomized controlled trials, these intervention pathways may serve as an adequate starting point in consultation with hematology. ■
References
Silverman MA. Immune Thrombocytopenia (ITP) in Emergency Medicine. Accessed June 22, 2021, 2021.
Cines DB, Bussel JB. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N Engl J Med. 06 2021;384(23):2254-2256. doi:10.1056/NEJMe2106315
Johnson & Johnson’s Janssen COVID-19 Vaccine Overview and Safety. Updated June 1, 2021. Accessed June 15, 2021.
Pfizer-BioNTech COVID-19 Vaccine Overview and Safety. Updated May 27, 2021. Accessed June 15, 2021, 2021.
Moderna COVID-19 Vaccine Overview and Safety. Accessed June 15, 2021.
Schoenmaker L, Witzigmann D, Kulkarni JA, et al. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int J Pharm. May 2021;601:120586. doi:10.1016/j.ijpharm.2021.120586
Heublein M, Gandhi M, Chiu C, Okamoto E, Karginov T, Williams P. The Curbsiders. #253 COVID-19 Vaccines with Dr. Monica Gandhi.
Pavord S, Lester W, Makris M, Scully M, Hunt B. Guidance from the Expert Haematology Panel (EHP) on Covid-19 Vaccine-induced Immune Thrombocytopenia and Thrombosis (VITT). 2021:7. 28 May 2021. Accessed 21 June 2021.
Kowarz E, Krutzke L, Reis J, Bracharz S, Kochanek S, Marschalek R. “Vaccine-Induced Covid-19 Mimicry” Syndrome: Splice reactions within the SARS-CoV-2 Spike open reading frame result in Spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. Research Square2021.
Lu L, Xiong W, Mu J, et al. The potential neurological effect of the COVID-19 vaccines: A review. Acta Neurol Scand. Jul 2021;144(1):3-12. doi:10.1111/ane.13417
Greinacher A, Thiele T, Warkentin T, Weisser K, Kyrle P, Eichinger S. A Prothrombotic Thrombocytopenic Disorder Resembling Heparin-Induced Thrombocytopenia Following Coronavirus-19 Vaccination. Research Square2021.
Greinacher A, Selleng K, Mayerle J, et al. Anti-SARS-CoV-2 Spike Protein and Anti-Platelet Factor 4 Antibody Responses Induced by COVID-19 Disease and ChAdOx1 nCov-19 vaccination. Research Square2021.
Arepally GM, Padmanabhan A. Heparin-Induced Thrombocytopenia: A Focus on Thrombosis. Arterioscler Thromb Vasc Biol. 01 2021;41(1):141-152. doi:10.1161/ATVBAHA.120.315445
Immune Thrombocytopenia. Accessed June 22, 2021.
Zeller B, Helgestad J, Hellebostad M, et al. Immune thrombocytopenic purpura in childhood in Norway: a prospective, population-based registration. Pediatr Hematol Oncol. 2000 Oct-Nov 2000;17(7):551-8. doi:10.1080/08880010050122816
Dominguez M. Immune Thrombocytopenia. Updated 09/25/2019. Accessed June 15, 2021, 2021.
Patriarcheas V, Pikoulas A, Kostis M, Charpidou A, Dimakakos E. Heparin-induced Thrombocytopenia: Pathophysiology, Diagnosis and Management. Cureus. Mar 2020;12(3):e7385. doi:10.7759/cureus.7385