A New Mode to Diagnose Pneumo(Peritoneum)
Almost reflexively, after determining a patient is “sick,” I find myself running to grab our ultrasound machine. The ability to get quick answers, with the bonus of low cost and no radiation risk to the patient, has spiked my interest to learn and use point-of-care ultrasound (POCUS), especially on our sickest patients with life-threatening diagnoses. For example, even before the portable x-ray machine rolls into the patient’s room, I can confidently determine whether a patient with shortness of breath has acute decompensated heart failure or a COPD exacerbation. However, I first had to learn to identify B-lines.
Recently, I came across an intriguing blog post in which POCUS was used to diagnose intra-abdominal free air (IFA). Now that I’ve learned the technique and signs, I am undoubtedly going to apply this to my next patient with acute abdominal pain, and hopefully, I will diagnose pneumoperitoneum.
Pneumoperitoneum
Pneumoperitoneum is an all-encompassing term for IFA inside the peritoneal cavity but outside the bowel lumen. Most often, patients will present with severe, sudden onset abdominal pain, associated with nausea and vomiting. The physical exam is variable, and ranges from mild to severe abdominal tenderness with distension, rebound and guarding.
There is a long list of causes for IFA (Table 1), and often the history is enough to narrow the differential diagnosis. Up to 85-95% of the time, IFA is caused by a perforated viscus or a defect in the gastrointestinal tract. These patients require emergent surgery, and a missed diagnosis is associated with high mortality. Non-operative etiologies include increased abdominal pressure (severe coughing, CPR, COPD) and iatrogenic causes (post-laparoscopic or open abdominal surgery). [1]
Table 1: Common causes of IFA requiring surgical intervention, based on location
Esophageal perforation
Risk factors: recent instrumentation, esophageal cancer, history of forceful vomiting
ROS: +chest pain, +dysphagia, +epigastric pain
Other clues: subcutaneous emphysema
Small bowel
Acute mesenteric ischemia
Risk factors: atrial fibrillation, CAD
History: postprandial pain, weight loss
Labs: elevated lactate
Other causes:
- Malignancy
- Meckel’s diverticulum
Perforated peptic ulcer
Gastric or duodenal ulcer
Risk factors: NSAID use, H. pylori
Colorectal
Toxic megacolon
Risk factors: C. difficile colitis, IBD
Other causes:
- Diverticulitis
- Appendicitis
- Malignanc
- Ischemic colitis
- Iatrogenic (post colonoscopy)
- Bowel obstruction
Imaging
Plain film radiography
Radiography alone is not sensitive enough to rule out IFA, and published sensitivities range between 55-85%. [2] As patients need to be positioned upright or on their side for at least 10 minutes, plain films may prove difficult in our critically ill patients.
Of upright and supine views, the former is more sensitive. Classically, you would look for air under the diaphragm; however, this is only seen in 60-80% of cases. [3] Some studies describe the ability of upright films to detect as little as 1 mL of free air,4 though this involves a great deal of technical precision and patience. If unable to obtain upright views, the supine left lateral decubitus view is the next best choice. Free air can be seen between the liver edge and the abdominal wall.
Subtle signs of IFA include the Falciform Ligament sign (when air collects in bilateral subphrenic spaces to reveal a linear density on the ventral surface of liver) and the Rigler sign (when air is visualized on both sides of the bowel wall). [5]
CT
CT is the imaging modality of choice as it has the highest sensitivity and specificity. It is excellent at detecting air in the retroperitoneal spaces, such as with perforated sigmoid diverticulitis. IV contrast is useful to detect fat stranding, bowel wall defects, or bowel wall thickening. In 90% of positive scans, air will be most pronounced at the site of the perforation. [6]
Unfortunately, CT is not always the wisest first choice in EDs due to considerations of vague presenting symptoms, risks of radiation, cost, and potential delays in overcrowded EDs.
Ultrasound
Comfort with the POCUS technique is an invaluable skill for emergency physicians for several reasons. A busy ED might have imaging delays with CT scanners occupied with trauma patients or no available patient transporters, and a bedside exam might save the day. Another advantage of POCUS is that it is a quick, low radiation option in patients who are “too unstable” to leave the department. It is also a more appropriate first choice in pregnant women and infants, in whom we want to reduce risks of radiation.
Ultrasound is more sensitive for free air than plain radiography. One study found ultrasound to have a 95% sensitivity in detecting pneumoperitoneum compared to 78% with plain films. [7] Additionally, ultrasound has improved ability to detect “indirect” findings of bowel perforation, such as decreased peristalsis and free fluid between intestinal loops. [8]
That being said, it is important to remember that POCUS is operator-dependent, and an average user might forget the subtleties of how to perform the scan or might not be familiar with the ultrasound findings altogether. POCUS is a “rule-in” test and is extremely useful as a screening test to expedite next steps. Given the significant morbidity and mortality associated with this diagnosis, a negative POCUS exam should not be interpreted as an absence of pathology.
Technique
This exam seeks to answer one simple question: Is there free air in the abdomen?
Probe selection
This depends on the patient’s body habitus. The higher frequency linear probe (10-12 MHz) is preferred in thin patients due to superior resolution. Unfortunately, this probe is limited by shorter depth penetration. Thus, in patients with more adipose tissue, the lower frequency curvilinear probe (3-5 MHz) will allow greater depth visualization. While Nazerian et. al. found improved specificity using the linear probe compared to the curvilinear probe (95.5% vs 81.8%), there was no significant difference in sensitivity. [2] An additional benefit is that the same probe can be used to perform several other scans simultaneously, such as the gallbladder, aorta or FAST.
Several scanning techniques have been published. Hefney et.al. describe scanning the patient in the epigastrium and in the RUQ, in both supine and left lateral decubitus positions. Deep inspiration was shown to enhance image acquisition. [9] Karahan et. al. also describe scanning the patient in the supine position, with the sagittal probe in the right paramedian epigastric area, and in the left lateral position with the transverse probe placed in the right mid axillary line, parallel to parallel to 8th and 10th intercostal spaces. Strong reverberations may shift with position change, and this is known as the shifting phenomenon. [10]
Scissor Maneuver
Karahan et. al. have described a novel and useful technique called the “scissor maneuver,” which can assist in identifying pneumoperitoneum. When pressure is applied to the caudal aspect of the probe, the reverberation artifacts disappear as the free air is pushed away. When pressure is released, the reverberation artifacts become apparent again. [10]
For a better visual idea of what the Scissor Maneuver looks like, read this blog post from EM Docs.
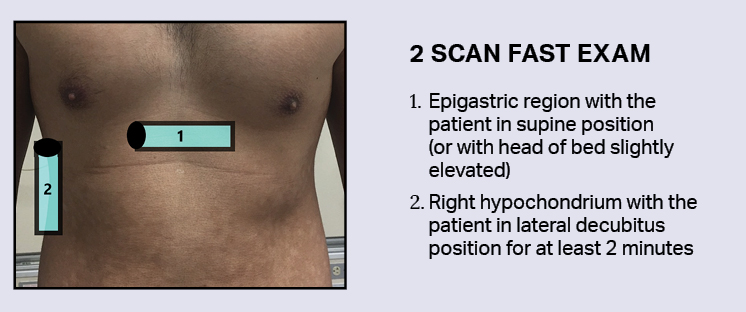
2 Scan FAST exam
Nazerian et. al. describe a quick 2-location exam, which has been shown to be equally efficacious as scanning five abdominal locations (epigastrium, right hypochondrium, left hypochondrium, umbilical, and right hypochondrium with patient in left lateral decubitus position). [2] The best two locations were found to be the epigastric region with the patient in supine position, and the right hypochondrium with the patient in lateral decubitus position for at least 2 minutes. [2] The goal of the 2-minute wait is to bring the free air anterior and close to the probe.
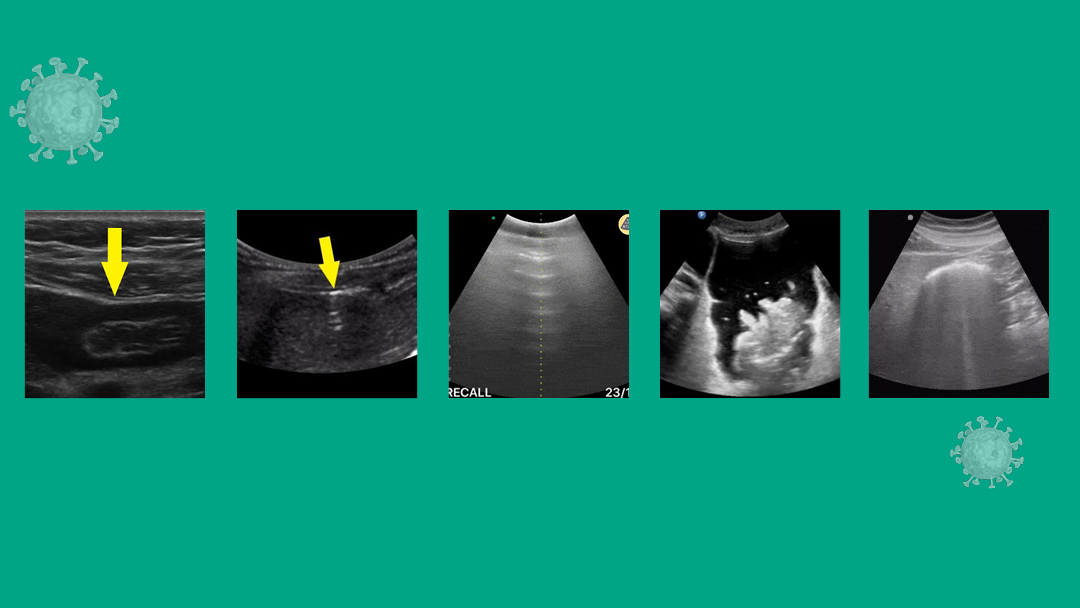
Signs
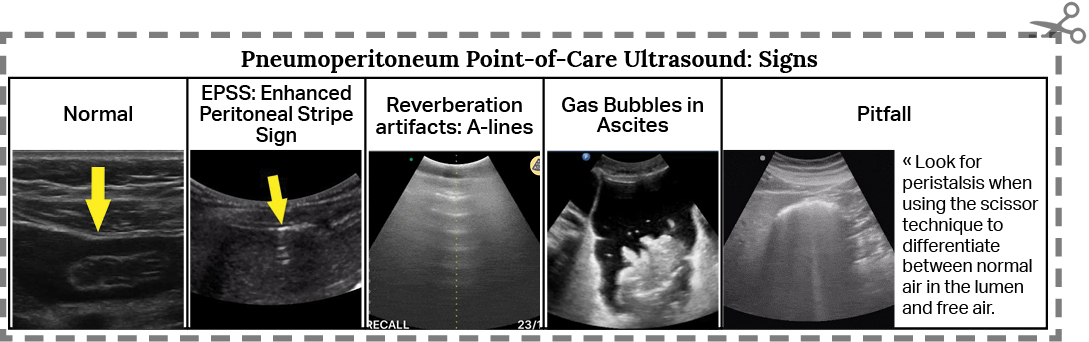
Four of the most well described POCUS signs in patients with IFA are: (a) the enhanced peritoneal stripe sign, or EPSS, (b) the presence of reverberation artifacts, (c) comet tails artifacts and (d) the presence of air bubbles in ascites.
(a) EPSS: Normally, the peritoneal stripe will appear as a thin, echogenic line between the anterior abdominal wall and underlying organs or peritoneal fluid. With disruption by air, the interface between the gas and underlying soft tissue scatters sound waves to result in an “enhanced” peritoneal stripe. [11,12] (Fig. 1)
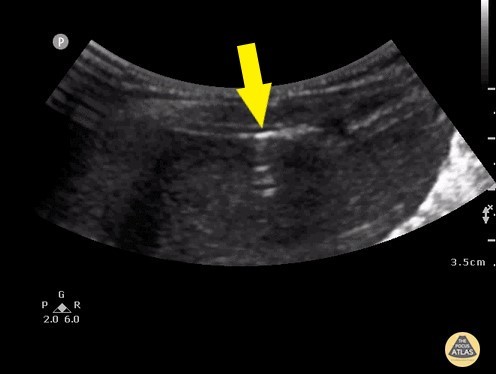
(b) Reverberation artifacts: Large amounts of free air can produce echogenic reflections known as reverberation artifacts. These appear similar to the “A-lines” seen by ultrasound in normal lungs. (Fig. 2)
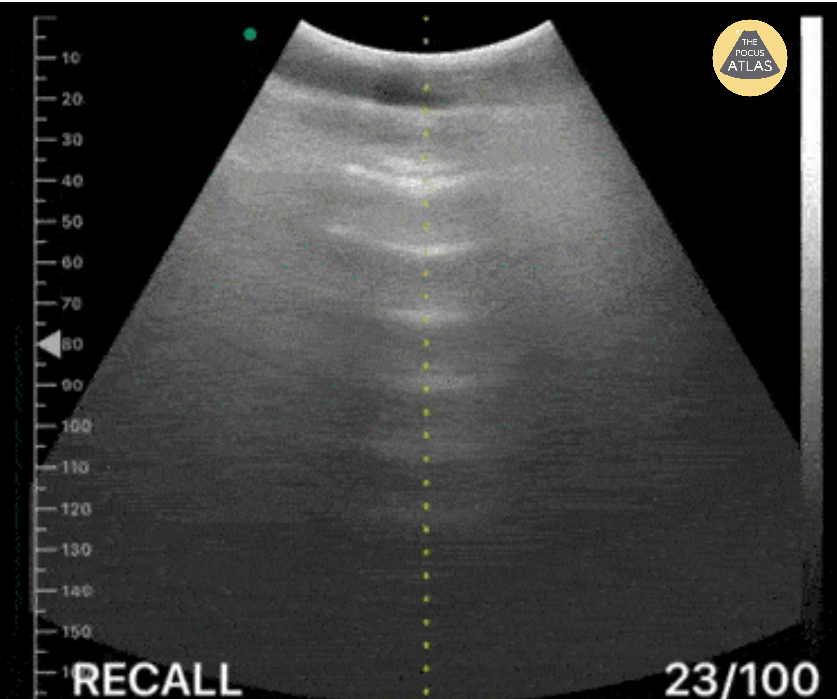
(c) Comet tail artifacts: Comet tails are essentially several echogenic reverberations stacked together to resemble a streak similar in appearance to a comet’s tail.
(d) Air bubbles in ascites: Often, free air is found with free fluid in the abdomen. Air bubbles can be seen clearly as hyperechoic, mobile “bubbles” floating in dark pockets of ascites. (Fig. 3)
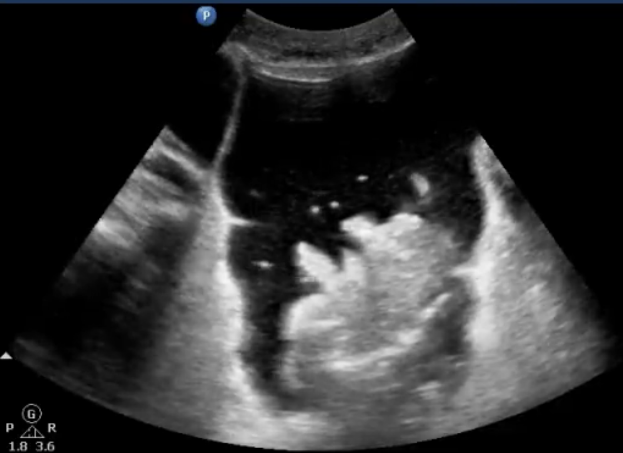
Pitfalls
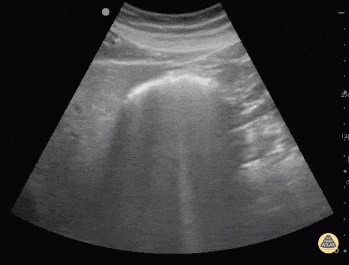 There are several pitfalls to remember. The most frequently encountered one is confusing air in the bowel (Fig. 4) with free air in the abdomen. The presence of peristalsis can help differentiate the two. Another way to differentiate both is by scanning in the right hypochondrium, where the solid liver will prevent bowel loops from causing confusion. While scanning in the right upper quadrant, take heed to not confuse normal reverberation artifacts in the lung with that caused by IFA. Normal lung artifacts will move with respiration and will be located above the peritoneal line. [9]
There are several pitfalls to remember. The most frequently encountered one is confusing air in the bowel (Fig. 4) with free air in the abdomen. The presence of peristalsis can help differentiate the two. Another way to differentiate both is by scanning in the right hypochondrium, where the solid liver will prevent bowel loops from causing confusion. While scanning in the right upper quadrant, take heed to not confuse normal reverberation artifacts in the lung with that caused by IFA. Normal lung artifacts will move with respiration and will be located above the peritoneal line. [9]
POCUS is commonly used in patients presenting with acute abdominal pain, especially if renal stones, gallstones or AAA is suspected. However, it is not utilized enough to diagnose IFA. Expanding our POCUS skills to include this diagnosis may prove invaluable in saving patients’ lives. Familiarity with the simple technique, signs and pitfalls is a bold move towards this. ■
Pneumoperitoneum.To.Go.

References
- Tanner, Tiffany Nicole, et al. “Pneumoperitoneum.” Surgical Clinics of North America, vol. 98, no. 5, 2018, pp. 915–932., doi:10.1016/j.suc.
- Nazerian, P, et al. “Accuracy of Ultrasonography for the Diagnosis of Pneumoperitoneum.” Critical Ultrasound Journal, vol. 7, no. S1, 2015, doi:10.1186/2036-7902-7-s1-a14.
- Williams, N. M., and D. F. Watkin. “Spontaneous Pneumoperitoneum and Other Nonsurgical Causes of Intraperitoneal Free Gas.” Postgraduate Medical Journal, vol. 73, no. 863, Jan. 1997, pp. 531–537., doi:10.1136/pgmj.73.863.531.
- Braccini, G., et al. “Ultrasound versus Plain Film in the Detection of Pneumoperitoneum.” Abdominal Imaging, vol. 21, no. 5, 1996, pp. 404–412., doi:10.1007/s002619900092.
- Hokama, Akira, et al. “The Falciform Ligament Sign of Pneumoperitoneum.” Journal of Emergencies, Trauma, and Shock, Medknow Publications, July 2011, www.ncbi.nlm.nih.gov/pmc/articles/PMC3162727
- Borofsky, Samuel, et al. “The Emergency Room Diagnosis of Gastrointestinal Tract Perforation: the Role of CT.” Emergency Radiology, vol. 22, no. 3, 2014, pp. 315–327., doi:10.1007/s10140-014-1283-4.
- Chen, S.-C., et al. “Ultrasonography Is Superior to Plain Radiography
in the Diagnosis of Pneumo-peritoneum.” British Journal of Surgery, vol. 89, no. 3, 2002, pp. 351–354., doi:10.1046/j.0007-1323.2001.02013.x. - Grassi, Roberto, et al. “Gastro-Duodenal Perforations: Conventional Plain Film, US and CT Findings in 166 Consecutive Patients.” European Journal of Radiology, vol. 50, no. 1, 2004, pp. 30–36., doi:10.1016/j.ejrad.2003.11.012.
- Hefny, Ashraf, and Fikri Abu-Zidan. “Sonographic diagnosis of intraperitoneal free air.” Journal of Emergencies, Trauma, and Shock, vol. 4, no. 4, 2011, p. 511.
- Karahan, Okkes Ibrahim, et al. “New Method for the Detection of Intraperitoneal Free Air by Sonography: Scissors Maneuver.” Journal of Clinical Ultrasound, vol. 32, no. 8, 2004, pp. 381–385., doi:10.1002/jcu.20055.
- 1Asrani, Ashwin. “Sonographic Diagnosis of Pneumoperitoneum Using the ‘Enhancement of the Peritoneal Stripe Sign.’ A Prospective Study.” Emergency Radiology, vol. 14, no. 1, 2007, pp. 29–39., doi:10.1007/s10140-007-0583-3.
- Indiran, Venkatraman, et al. “Enhanced Peritoneal Stripe Sign.” Abdominal Radiology, vol. 43, no. 12, 2018, pp. 3518–3519., doi:10.1007/s00261-018-1628-7.
This article is part of the following sections: