Poison Control: 2021 Brodifacoum-Contaminated Synthetic Cannabinoid Outbreak: Overview of Identification and Management of the Exposed Patient
What happened during the 2021 brodifacoum outbreak?
In December 2021, patients started presenting to emergency departments across Hillsborough County with profound and persistent coagulopathies in which standard treatments were inadequate. It was later determined that these patients had been exposed to brodifacoum-contaminated synthetic cannabinoids. To date, there have been a total of 55 cases resulting in six deaths. The Florida Poison Information Center Tampa collaborated with local and state health departments and hospitals to develop a course of action for the identification and management of these patients.
What is brodifacoum? What are synthetic cannabinoids?
Brodifacoum belongs to a class of agents that was developed to overcome warfarin resistance in rats. Brodifacoum is a vitamin K antagonist and works similarly to warfarin by inhibiting vitamin K 2,3-epoxide reductase. [1] Inhibition of this enzyme inhibits the regeneration of vitamin K 2,3-epoxide to vitamin K. Vitamin K is an integral cofactor for factors VII, IX, X, II and proteins C and S.1,2,3 By preventing its activation, the coagulation cascade is impaired, thus resulting in coagulopathy. These rodenticides are referred to as “superwarfarins” or long-acting anticoagulant rodenticides (LAARs). Brodifacoum is the most potent agent in this class; it is over 100x more potent than warfarin and can last from 2-12 months. [2,4]
Synthetic cannabinoids are a class of lab-derived cannabinoids that are full agonists of the Cannabinoid receptor 1 (CB1) and CB2 receptors throughout the body, but more specifically in the brain. Synthetic cannabinoids are commonly referred to as “K2”, “Spice” or “Cloud 9” and are a common drug of abuse. [5]
How to identify patients possibly exposed to brodifacoum
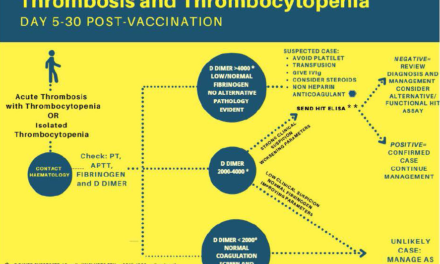
Most commonly, patients exposed to brodifacoum will present to the emergency department with unexplained bleeding. Hematuria is the most common type of bleeding seen, but other common types of bleeding include epistaxis, vaginal, and gastrointestinal bleeding. Patients will also have unmeasurably high PT/INR levels. In addition, most exposed patients have a history of drug use or reported using synthetic cannabinoids in the days to weeks prior to their presentation. Laboratory tests to detect brodifacoum are not readily available in the acute setting, so the diagnosis of brodifacoum exposure is clinical in nature and dependent on a high index of suspicion. Contact your local poison center if you are suspecting a patient exposed to brodifacoum.
What is the approach to treatment?
A baseline CBC and PT/INR should be immediately obtained in addition to consultation with your local poison control center.
Initial treatment may include both IV and high-dose oral vitamin K, fresh frozen plasma (FFP), or if the patient is critically bleeding, 4-factor prothrombin complex concentrate (PCC). After initial treatment and stabilization, serial CBCs and PT/INR values are required at least every 12 hours monitoring. Initial treatment is not curative, and these patients will all require admission until normalization of their PT/INR and an appropriate outpatient dose of vitamin K is established. As mentioned previously, brodifacoum has a very long duration of action and exposed patients require treatment with high doses of oral vitamin K for an average of 6-9 months.
What are the long-term effects?
Due to the long duration of action of brodifacoum, exposed patients require continued treatment for an average of 6-9 months. They will require close outpatient monitoring and medication administration. One major barrier to the long-term treatment of these people is medication non-compliance, which may result in the redevelopment of coagulopathy and bleeding requiring representation to the hospital.
When managing a suspected brodifacoum exposure, healthcare professionals at the Florida Poison Information Center Network are available at 1-800-222-1222 to answer questions concerning to, or assist in the management of, this or any other toxic exposure. ■
References:
- Brodifacoum. In: IBM Micromedex POISINDEX (electronic version). IBM Watson Health, Greenwood Village, Colorado, USA. (cited: March 1, 2022).
- H.A. Spiller. Brodifacoum,Editor(s): Philip Wexler, Encyclopedia of Toxicology (Third Edition), Academic Press, 2014, pages 543-545.
- Kelkar AH, Smith NA, Martial A, Moole H, Tarantino MD, Roberts JC. An Outbreak of Synthetic Cannabinoid-Associated Coagulopathy in Illinois. N Engl J Med. 2018 Sep 27;379(13):1216-1223.
- Gunja N, Coggins A, Bidny S. Management of intentional superwarfarin poisoning with long-term vitamin K and brodifacoum levels. Clin Toxicol (Phila). 2011 Jun;49(5):385-90.
- Mills B, Yepes A, Nugent K. Synthetic Cannabinoids. Am J Med Sci. 2015 Jul;350(1):59-62.
This article is part of the following sections: